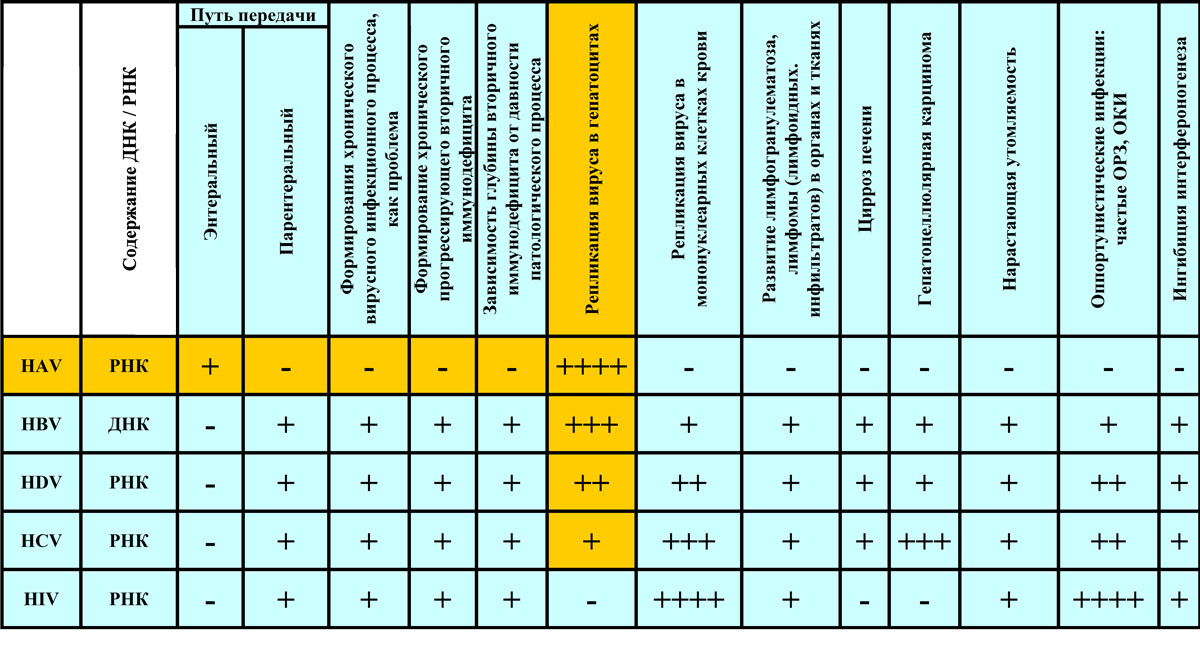
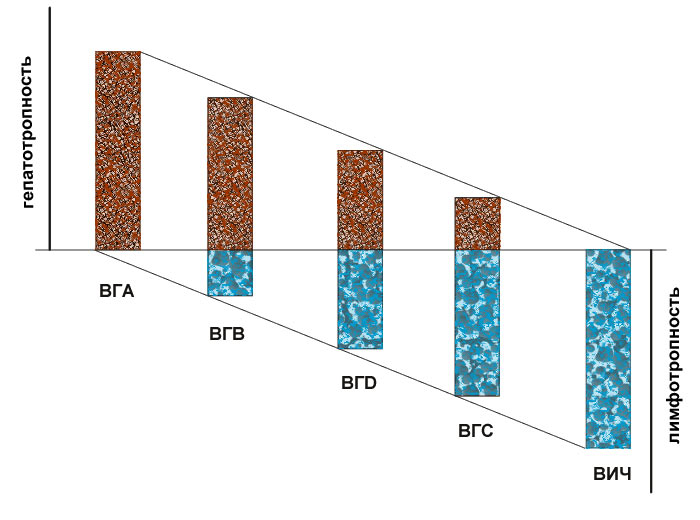
The present method of detecting lymphotropic viruses is intended for:
- to increase the reliability of determining the infection of biological media with hepatitis B virus (HBV), hepatitis C virus (HCV) or human immunodeficiency virus (HIV) with lymphotropism; - to exclude false negative reactions when testing donated blood and patients for infection with HBV, HCV or HIV; - to detect HBV, HCV or HIV in biological material (donor blood, plasma and other blood derivatives) with a concentration of viral particles below the sensitivity threshold of the ELISA and PCR method; - to assess the viability of viruses after exposure to chemical and physical antiviral factors developed and offered by manufacturers in order to inactivate viruses in practical healthcare.
This method has no analogues.
Presentation of the method

VI METHOD FOR ASSESSING IN VITRO VIABILITY OF LYMPHOTROPIC VIRUSES HBV, HCV AND HIV.
- Obtaining suspension of HBV, HCV or HIV. To obtain suspension of viruses in patients infected with HBV, HCV or HIV monoinfections, with a previously known high viral load, blood is taken from the ulnar vein. Virus-containing serum is isolated from whole blood, which is subjected to quantitative PCR examination to confirm the presence of HBV, HCV or HIV and to determine the titer of viruses. The virus-containing serum is stored frozen in a minus refrigerator at a temperature below - 25 ° C.
- Obtaining suspension of lymphocytes of a healthy person.
- Healthy human volunteers are tested for infection with HBV, HCV and HIV by the ELISA method. The tests use lymphocytes of healthy people only with negative test results;
- To obtain sufficient amount of lymphocyte suspension, blood is taken from a healthy person (volunteer) in the morning on an empty stomach from the ulnar vein in an amount of 30-40 ml. Next, 7-8 ml of blood is transferred to centrifuge tubes containing 2 ml of saline solution with 3 drops of heparin. The mixture in the test tube is thoroughly mixed;
- The isolation of lymphocytes from whole heparinized blood is carried out by separation in the ficoll-verografin gradient with a density of d = 1.077 g / ml according to the method of F.Y. Garib et al. (1995). To do this, 2 ml of ficoll-verografin gradient is poured into clean centrifuge tubes, heparinized blood is carefully layered on its surface and the tubes are centrifuged at 1500 rpm. During centrifugation, all shaped blood elements, except lymphocytes, penetrate through the thickness of the ficoll-verografin gradient and are located under it. The blood plasma remains above the gradient. In the thickness of the gradient, a kind of cloudy ring ("cloud") is formed, consisting of a suspension of lymphocytes. This ring with lymphocytes is carefully sucked off with a pipette and transferred to a clean centrifuge tube;
- Next, a 2x-3x washing of lymphocytes in 10.0 ml of saline solution is carried out. After the last centrifugation, the filler liquid is removed. Sediments containing lymphocytes are diluted in 500 ml of saline solution and mixed. A suspension of lymphocytes from all tubes merge into one tube. The suspension of lymphocytes can be stored for no more than 1 day at a temperature of + 4oC.
In vitro evaluation of the ability of HBV, HCV or HIV viruses to penetrate the cytoplasm of human lymphocytes.
- HBV, HCV or HIV containing plasma is removed from the minus refrigerator, thawed at room temperature.
- The equal volume (300 ml each) of virus-containing plasma and lymphocyte suspension is transferred to a clean test tube using an automatic pipette, the contents are mixed and put for incubation (incubation of viruses with invitro lymphocytes) in a thermostat at a temperature of +37°C for 6-8 hours. The contents of the test tube are mixed by shaking every 1.5-2 hours. During incubation, only viable viruses that exhibit cytopathogenic effects penetrate into the cytoplasm of lymphocytes.
- Washing of lymphocytes from plasma. Next, the test tube is removed from the thermostat. 6-8 ml of saline solution is added to it, mixed and centrifuged at 1500 rpm/min. There is a deposition of lymphocytes to the bottom of the tubes. The filler fluid is removed. Next, a 2x-3x washing in saline solution and precipitation of lymphocytes is performed.
- After the last centrifugation and removal of the infusion fluid, the suspension of lymphocytes (sediment) is diluted in 500 mcl of saline solution and transferred to the epindorph.
- After that, for the destruction of lymphocytes, epindorph is placed in the freezer of a household refrigerator for 17-18 hours. With slow freezing, the destruction of lymphocyte membranes occurs.
- The supraventricular fluid from the epindorph is sucked out and subjected to quantitative PCR examination for the detection of DNA or RNA viruses in the cytoplasm of lymphocytes that were previously contained in the blood plasma from patients. Evaluation of results.
- A positive result of a PCR study for the presence of RNA or DNA viruses in the contents of the cytoplasm of lymphocytes indicates the preservation of the viability of viruses, that is, their ability to penetrate and persist in human lymphocytes in vitro.
- A negative result of PCR examination for the presence of RNA or DNA viruses in the contents of the cytoplasm of lymphocytes indicates the loss of viability (inactivation) of viruses (after exposure to antiviral factors of chemical or physical nature), that is, the loss of their ability to penetrate and persist in human lymphocytes in vitro.
VII THE RANGE OF APPLICATIONS OF THE METHOD FOR DETECTING AND ASSESSING THE VIABILITY OF LYMPHOTROPIC VIRUSES HBV, HCV AND HIV
- Blood transfusion stations: to increase the reliability of detection of lymphotropic hepatitis B (HBV), hepatitis C (HCV) and human immunodeficiency virus (HIV) in biological media (donor blood, plasma and other components) containing viruses in concentrations below the threshold of sensitivity of ELISA or PCR methods.
- Diagnostic laboratories: to increase the reliability and exclude false negative results when testing for HBV, HCV or HIV infection in the blood of donors or patients with virus content in concentrations below the threshold of sensitivity of ELISA or PCR methods.
- Pharmaceutical companies: : to assess the viability of HBV, HCV or HIV viruses after exposure to chemical and physical antiviral factors developed and offered by manufacturers in order to inactivate viruses at various facilities.
- The method makes it possible to eliminate errors in the diagnosis of patients, when testing donated blood and its components for infection with lymphotropic viruses HBV, HCV or HIV. The method makes it possible to objectively assess the effects of chemical or physical factors on the viability (pathogenicity) of lymphotropic viruses.
- The method of detecting and evaluating the viability of lymphotropic viruses has no analogues.
- This method is registered by the Ministry of Health of the Republic of Uzbekistan.
Author: Gulyamov Nariman Gulyamovich, Doctor of Medicine, Professor